-
Australia Online Casino Dollar 5 Deposit
Best Casino in Blackhawk AU
Live Pokies with Welcome Bonus Low Deposit
Ultimate Pokies Hack for Mobile Phone
Moonee Valley Racing Club Pokies
Gambling Times AU
Play Coyote Free Top Pokies
Best Australia Online Casinos
Deposit Casino Pokies Real Money Options
List of AU Online Casinos A to Z
research
Cytoskeletal Mechanobiology
Systems Biomechanics
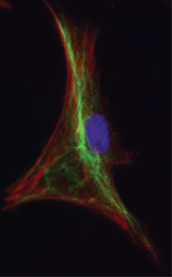

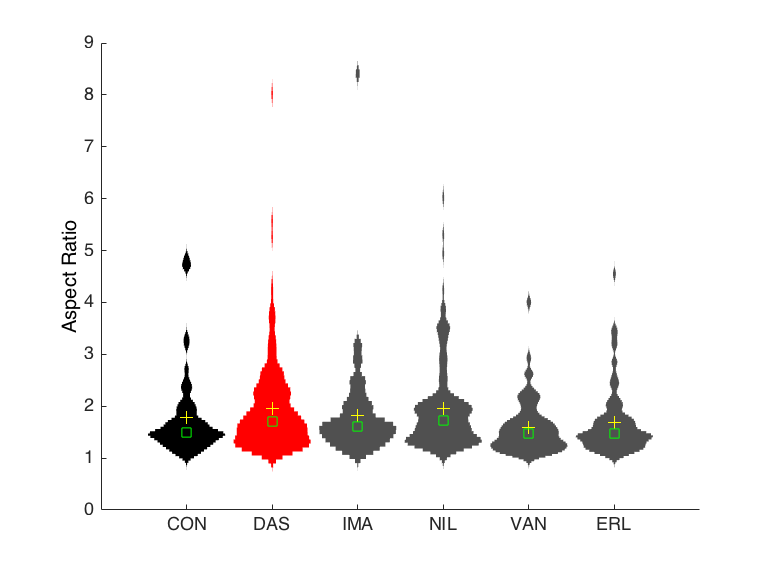
How the interaction of physical forces and biochemical processes affects basic cell biology still eludes most scientists. We study systems-level regulation of cell and tissue biomechanics to understand its role in progression of complex diseases, and to discover new drug targets and to design novel therapeutic strategies based on mechanobiological principles. We are specifically interested in proteins that form the focal adhesion complex and actin-associated proteins that shape the mechanobiological information processing capacity of the cell. In addition to playing critical structural roles, these crosslinking and adapter proteins modulate mechanotransduction through spatial segregation of signaling proteins. We use proteomics to identify key functional proteins within the cytoskeleton or the adhesome and utilize classical cell biological as well as bioengineering methods to characterize the complex role they play in pathophysiology. We have recently showed how multiple nested network motifs controlled expression and localization of the actin-crosslinking protein, synaptopodin, in kidney podocytes. Our findings have been featured as the cover story on Science Signaling.
Kidney Tissue Engineering
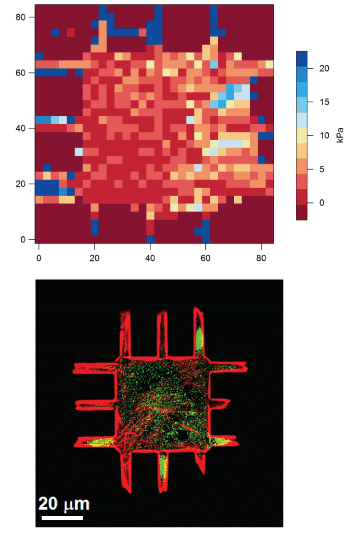
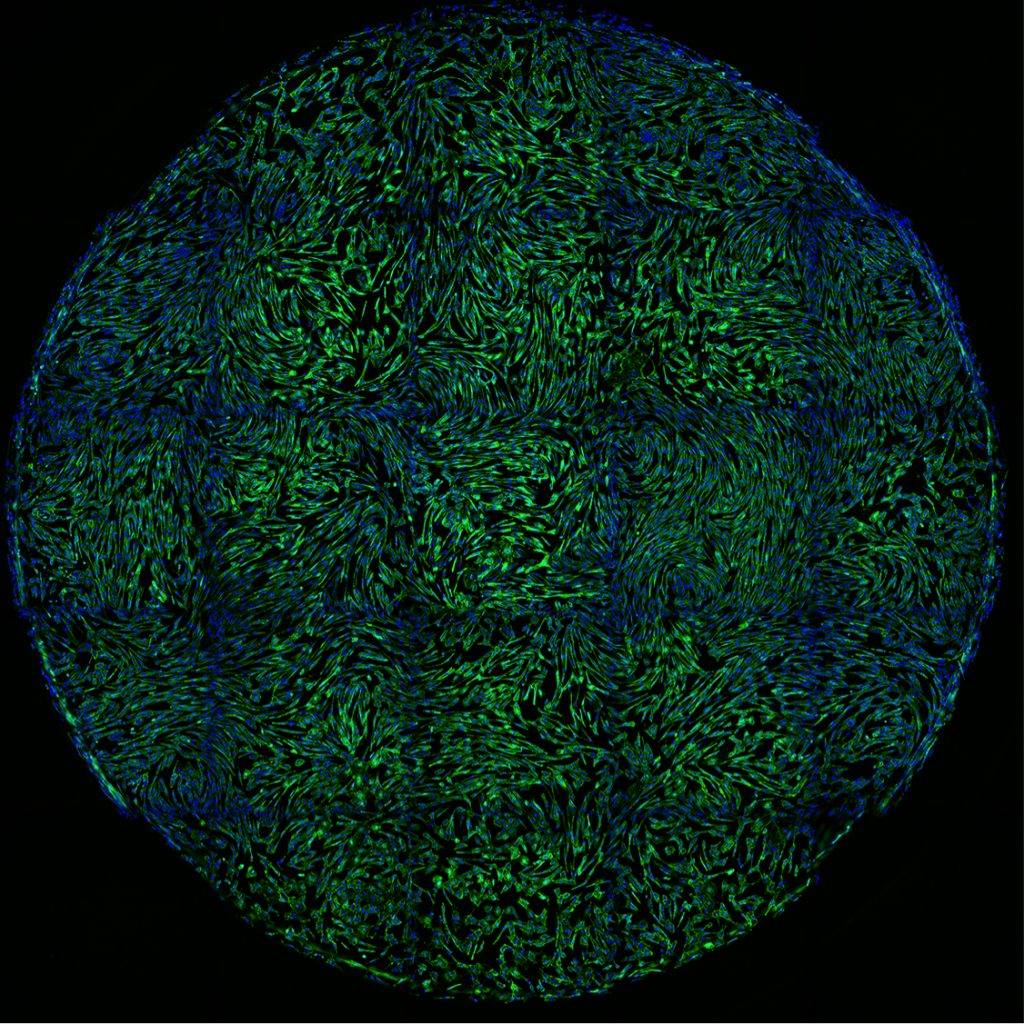
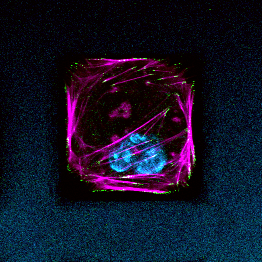
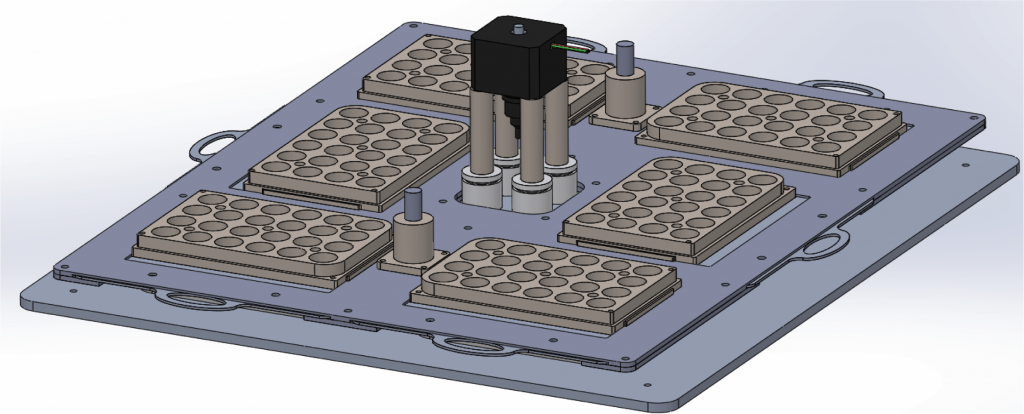
We combine nanotechnology, functional tissue engineering, and multiple-omics methodologies to study kidney biology and glomerular disease mechanisms. Despite the functional importance of their specialized morphology, isolated glomerular epithelial cells, or podocytes, do not exhibit any geometric hallmarks of their in situ morphology. We use innovative microfabrication techniques to construct long-term culture substrates that induce morphological remodeling of kidney podocytes into arborized, or branched, shapes. This geometric remodeling leads to functional specialization of peripheral projections where filtration proteins are translocated into the periphery of the cell. This phenomenon cannot be observed in immortalized podocytes cultured on regular, or unpatterned, surfaces. This phenotypic specialization can be used as a proxy for podocyte differentiation, which enabled us to develop a kidney-on-chip platform. Using induced pluripotent stem cells (iPSCs) to generate adult human podocoytes, we developed a high-content screening system that utilizes microengineered surfaces to quantitatively characterize cytoskeletal and biomechanical drug responses in kidney podocytes.
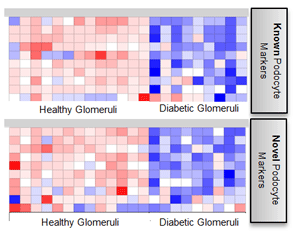
Biophysical Mechanisms of Nephrotoxicity